Metabolic health is imperative to overall wellness, affecting how your body processes energy, manages weight, and maintains vital functions. Metabolic health is characterized by optimal levels of blood sugar, triglycerides, HDL cholesterol, blood pressure, waist circumference and is crucial for reducing risks associated with heart disease, diabetes and stroke. Having clinically good metabolic health entails efficient nutrition to energy conversion for cellular turnover, which can further translate to improved mood, anxiety, skin health and memory. We can view metabolic health as the underlying factor for a wide range of lifestyle diseases.
The microbiome is of great importance when it comes to disease prevention, resolution and health maintenance. Each person’s microbiome is entirely unique, with trillions of organisms working to maintain host energy homeostasis. Homeostatic maintenance includes immune system modulation, which is directly tied to metabolic health. Microbial species in varying abundances within the microbiome have been linked with overall metabolic health, including the pathogenesis of common disorders like Obesity, Type 2 Diabetes, Malnutrition and others. The gut microbiota in particular are responsible for modulating gut physiology and motility, which is the movement of food through the digestive system from the mouth to exiting the body. In the host digestive tract, microbiota aid in the digestion of certain nutrients that contribute to energy production and further availability for use, such as for regenerative cellular processes and glucose homeostasis. Gut interactions drive biological processes such as the bacterial production of short-chain-fatty-acids (SCFAs), such as butyrate, to stimulate gut motility and host immunity, as well as metabolism. SCFA production has also been linked with reduced inflammation and positive metabolic outcomes. One of the more common species of bacteria that produces butyrate is Faecalibacterium prausnitzii, one of the most abundant species in the gut.
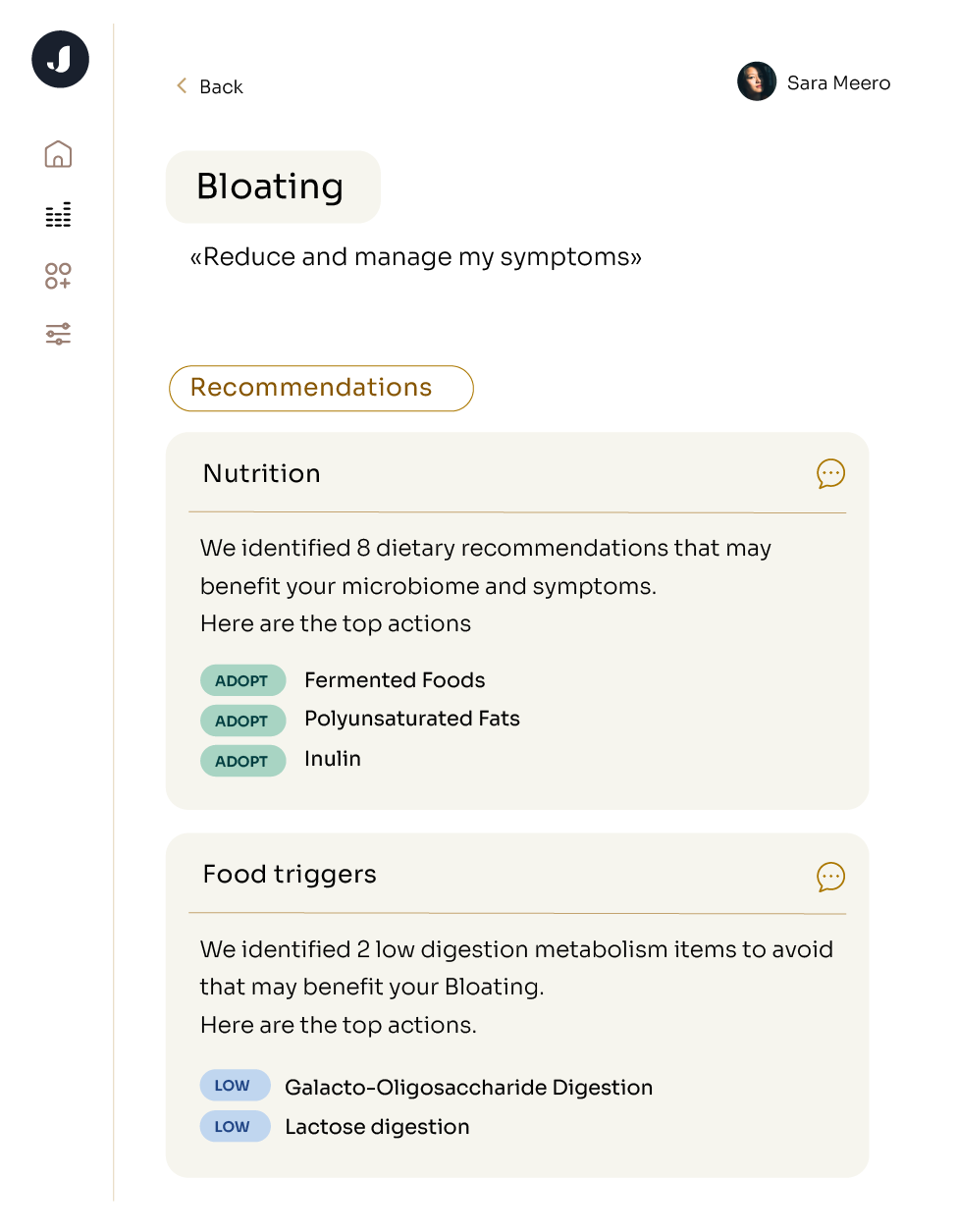
Jona’s microbiome analysis specifically examines the link between your microbiome and these metabolic conditions:
Hypertension and its comorbidities are commonly known as the cumulative disease Metabolic Syndrome. The key aspect of Metabolic Syndrome is insulin resistance, which can lead to diabetes. Another central link between hypertension and metabolic syndrome is obesity, where increased levels of adipose tissue that secrete adipocytokines produce arterial hypertension. What we see now is that approximately 120 million individuals in the United States are affected by hypertension, meaning that understanding and addressing these interconnected factors is crucial for effective metabolic health management. Recent research has highlighted the role of gut microbiota in this context, with studies indicating the distinct difference in microbial composition in healthy individuals vs. those with obesity. Gut microbiome activity contributes heavily to prediction of individual variation in glycemic response in adults
Prediabetes, characterized by elevated blood glucose levels, signals an increased risk of developing type 2 diabetes and can also heighten the likelihood of heart disease and stroke. Prediabetes manifests through elevated blood glucose levels, or hyperglycemia, serving as a clear indicator of compromised metabolic health. XXX Recognizing how this early indicator can unveil the critical risks of advancing to type 2 diabetes, heart disease, and other comorbidities. Recent research has uncovered that changes in specific bacterial strains within the gut microbiome can significantly elevate the risk of developing type 2 diabetes. For example, a decrease in beneficial strains like Bifidobacteria and Akkermansia muciniphila, combined with an overgrowth of bacteria such as Bacillota (Firmicutes), that can disrupt glucose metabolism and increase systemic inflammation. These organisms can be adjusted for each individual with a tailored approach of diet and lifestyle. If successful, prolonged individualized dietary control of the blood sugar levels after eating (Postprandial glycemic responses- PPGR) can be useful in controlling, improving, or preventing a set of disorders associated with chronically impaired glucose control, including obesity, diabetes, elevated triglycerides and other metabolic issues. PPGR has now been associated with genetics and body composition as well as the microbiome, which has allowed researchers to generate accurate predictions on the effects of different diets, since glycemic responses vary greatly between individuals. A 2021 study claims that a person’s glycemic response depends on individual traits, including both their anthropometrics (body size and form) and the activity of their specific gut microbiomes.
Building on our understanding of the gut's role in metabolic health, recent research reveals a compelling link between gut microbiota changes and autoimmune thyroid disorders like Hashimoto's disease. Hashimoto's, an autoimmune condition where the body attacks its own thyroid gland, often results in hypothyroidism and a range of metabolic issues. Emerging evidence shows that specific changes in gut bacteria, such as reduced levels of strains like Akkermansia muciniphila and Lactobacillus, alongside increased bacteria like Proteobacteria and Bacillota (Firmicutes), can intensify systemic inflammation and disrupt immune function. Research has shown that people in developed countries are more likely to be affected by diseases like Hashimoto’s disease and other autoimmune disorders. Evidence also points towards varied gut microbiomes having a negative effect on genesis and progression of autoimmune thyroid disorders. These microbial shifts contribute to a heightened inflammatory state and altered immune responses, potentially aggravating thyroid dysfunction. Jona’s technology works to identify your personal association with Hashimoto’s disease, and gives you the tools to ensure you are maintaining the best possible you — via your microbiome.
Issues stemming from metabolic health can lead-to or even exacerbate sleep issues, such as Obstructive Sleep Apnea. This occurs due to increased body weight/adiposity, and is perpetuated further by physiological mechanisms. When taking a closer, microbial-level look, we see that dominant species reveal themselves as being related to the pathogenesis of and occurrence of OSA. In patients with severe OSA, enriched levels of Fusobacterium and Megamonas are observed, deviating from healthy controls.Those same species are also related to biomarkers such as glucose levels and BMI – hallmarks of metabolic health. What Jona can help you deduce is that these bacterial level changes are involved in the pathophysiology of systemic inflammation and other metabolic comorbidities that are associated with OSA. Ever wonder how some individuals can function well on little sleep while others require more hours to operate? Evidence suggests a positive correlation between sleep efficiency and composition of gut microbiota from childhood through adulthood. Studies found vastly different genera of bacteria in children with high total sleep duration vs. low total sleep duration. In addition, a 2023 case study found that the severity of OSA was related to structural differences seen in the fecal microbiome, which varied by individual.