The gut and the brain speak to each other using electrical and chemical signals. Electrical signals are transmitted through a vine-like structure called the vagus nerve, which loops in other central organs and manages involuntary processes like gastric emptying, breathing, and heart rate. Chemical signals pass through the bloodstream until they reach their target receptors, where they can initiate signaling cascades passing messages to other organs. These signals allow your brain and gut to work as a team.
Your gut-brain axis is functioning in the background 24/7 to keep digestion in step with the body’s many other priorities. Information about food cravings, hunger and satiety, palatability, and pain travels from the gut to the brain through the enteric nervous system, the network of sensory capabilities inside your gut. Once this information arrives at the brain, networks including the hypothalamic-pituitary-adrenal axis help to triage and take action on these signals.
A few examples of the gut-brain partnership: a molecule called ghrelin, the ‘hunger hormone,’ tells your brain when you’re ready to eat and mediates the motility of food through the gut. Or, when you’re feeling anxious before a stressful event, your gut-brain axis reroutes blood away from the gut toward the brain, which can cause a loss of appetite. Together, the brain and gut systems allocate the body’s energy resources to grow, develop, and respond to external stimuli.
Your small and large intestines house the densest collection of human immune cells in the body. The gut’s resident immune cells, called macrophages, regulate inflammation and can even mediate neuronal loss.
However, the immune-boosting properties of the gut extend to the microbiome, as well. Gut microbial species such as B. fragilis and C. leptum can produce chemicals that upregulate immune pathways. (Importantly, upregulation of the immune system is not always beneficial and can contribute to inflammation or autoimmune disease over time, but it is critical for defense against pathogens.) These chemicals also reach the brain’s immune cells, called microglia, where they communicate information about inflammation and infection. On a more mechanical scale, the microbiome helps maintain the integrity of the gut walls, which creates a physical barrier against abdominal infections and reduces the likelihood of leaky gut syndrome. A healthy gut microbiome can support a strong immune system.
Maintaining a healthy gut microbiome can help promote mental health, just as managing your emotional health and stress levels can aid digestion. This often happens on a molecular level given the range of important neurotransmitters produced in the gut.
One interesting example connecting the microbiota, gut, and brain is that of tryptophan, one of 20 amino acids found in the proteins that we eat, and one of the few that the body cannot create itself. You may be familiar with tryptophan’s role in mediating the sleep-wake cycle; it also helps with gut motility and nutrient absorption. However, tryptophan is also a precursor to serotonin, a key neurotransmitter that is often associated with positive mood, and more than 90% of the body’s serotonin is produced by gut cells. This process is facilitated by the gut microbiota. Diminished tryptophan conversion has been observed in conditions such as depression and IBD, which have their own patterns of gut dysbiosis, spurring further research on the link between neurotransmitters and disorders of the gut-brain axis. Tryptophan is one of many such molecules with fascinating journeys through digestion, underscoring the complexity of the gut and its interactions with chemicals relevant for mental health.
Beyond digestion and cognition, the gut-brain connection loops in several other body systems. One example is the endocrine system, responsible for hormone signaling. The short-chain fatty acids produced by gut microbiota mediate release of hormone-like molecules such as GLP-1, and hormonal conditions such as polycystic ovary syndrome often co-occur with dysbiosis.
Permeability of the gut walls is another key mediator of the gut-brain pathway. Specific molecules produced by gut bacteria can also have broad effects on permeability and, as a result, inflammation elsewhere in the body. Sometimes, the bacteria themselves help maintain the cell wall: for example, B. infantis produces a beneficial protein that helps to maintain the tight junctions between cells. Other times, bacteria that escape from the digestive tract through weakened gut walls can wreak havoc elsewhere, and elevated intestinal permeability has been noted in nervous system disorders such as multiple sclerosis.
Given the broad reach of the gut-brain axis, it’s likely that many conditions are somehow related to this powerful link. Let’s spotlight a few here: research indicates that there may be common signatures in the gut microbiomes of people with Alzheimer’s disease and that inflammation through the gut-brain pathway could be critical in the development of this condition. Another example can be observed in individuals with autism spectrum disorder, who are more likely than the general population to experience GI concerns and tend to have higher levels of pro-inflammatory gut bacteria. As new research continues to emerge, our understanding of the link between microbiota, gut, and nervous system is rapidly evolving.
Neurological disorders including multiple sclerosis, schizophrenia, amyotrophic lateral sclerosis, and more have been linked to the gut. While the relationship between these diseases and gut dysbiosis is still being investigated (and, importantly, has not yet been proven causal in humans), clear patterns are beginning to emerge. Jona’s microbiome profiling can pick up on whether your microbiome matches the known signatures for any of these conditions.
IBS, or irritable bowel syndrome, merits a particular mention as some doctors classify it as a disorder of gut-brain interaction. It’s common for stress to trigger IBS flares, or short periods of worsening symptoms, and psychological conditions such as anxiety and depression occur at higher rates among those with IBS than the general population. The exact mechanisms governing the brain-gut axis in IBS are a topic of active research, but the presence of a link is clear. As a result, IBS experts explicitly mention stress management in treatment plans, and some studies even show that cognitive behavioral therapy can be effective in managing symptoms of IBS.
Your clinician will tailor treatment strategies to you, but strategies may include a gut microbiome profile, a medical history of your symptoms, a dietary journal, or even speaking with a counselor. Medication, probiotics, and nutritional supplements may help as well. If you’re interested in taking the first step towards understanding your gut and its association to your mental health, sign up for Jona here.
Your gut microbiome refers to the trillions of microbes that live in your small and large intestine, where they break down food and produce beneficial chemicals. The balance of microbial species in each person’s gut is unique and changes over time.
Many factors influence the makeup of your gut, starting as early as birth. Infants born vaginally are first colonized by bacteria originating from the mother, whereas C-section infants display different microbiome patterns even months after birth. As children mature, factors including emotional stress, antibiotic use, and diet are all important in shaping the microbiome. In adults, lifestyle choices as diverse as quitting smoking, living with a new roommate, and altitude exposure can all impact the gut microbiome.
Your gut microbiome lives near the end of your digestive system in the small intestine and colon. As a result, only a portion of your diet makes it all the way through your system to feed these microbes. Simple sugars and processed foods are broken down higher in the digestive tract, so it’s up to more durable foods high in indigestible dietary fiber to survive the journey to your gut microbiome, where they are converted into beneficial short-chain fatty acids. Scientists call these tough-to-digest substances “microbiota-accessible carbohydrates”, and they’re found in foods like beans, greens, and whole grains.
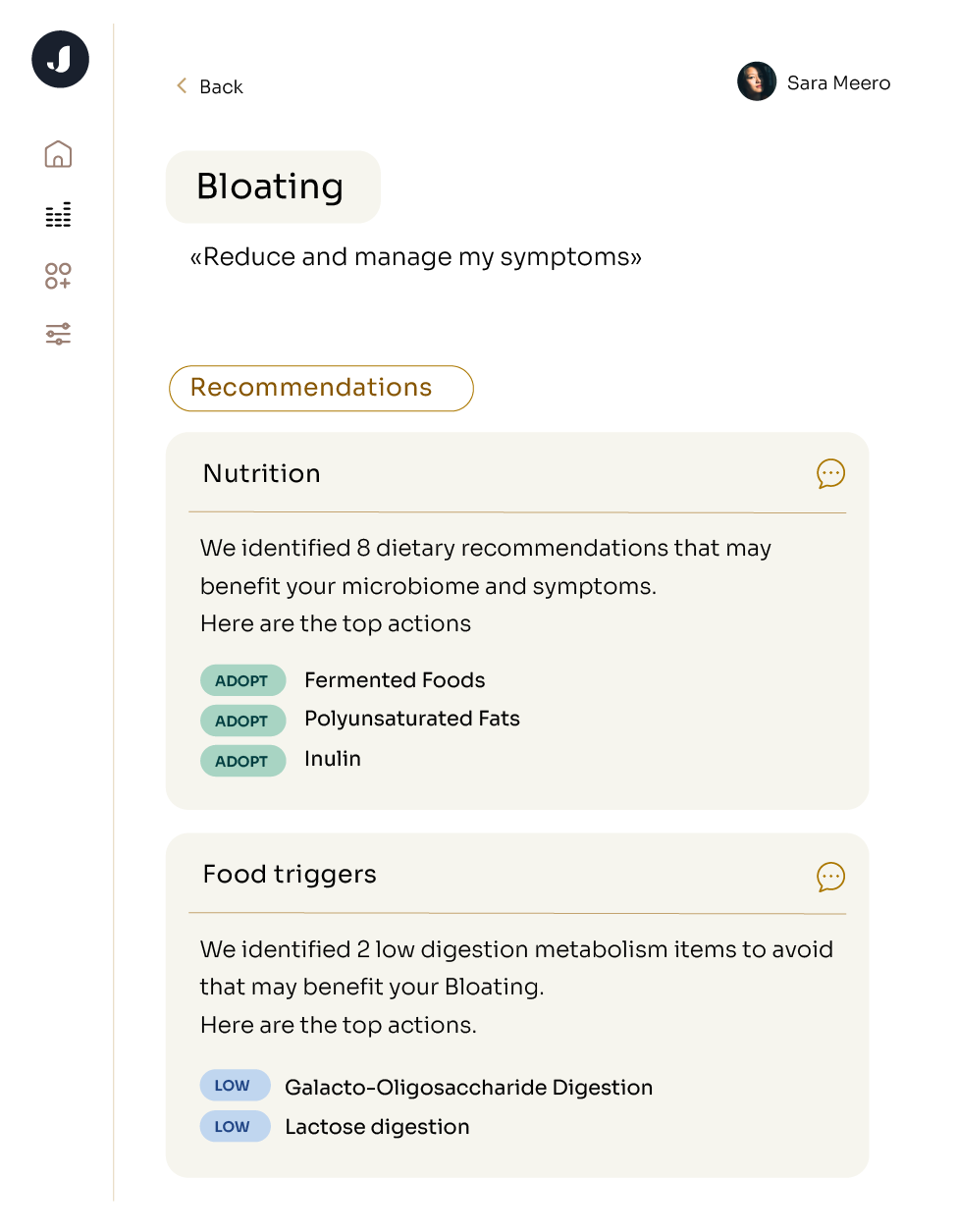
While a high-fiber diet is beneficial for many, it may not work for everyone. Each person’s gut is unique, and so is the balance of nutrients that will best nourish their microbiome. Jona’s at-home microbiome profiling can shed light on your current gut composition and the optimal dietary recommendations for you.
Diet and mood are intimately connected. We know that foods like blueberries and dark chocolate have been shown to improve mood and cognitive performance in various clinical trials, and some probiotics have been found to have similar effects. Please note that probiotics may not be right for everyone - check out our previous blog post addressing some of the controversy in this field. Should you feel a probiotic is right for you, our purchasing guide can be found here. Beyond the specifics of your diet, research suggests that eating a wide variety of foods can give your gut the chance to produce a broad panel of metabolites that can improve overall health.